44+ calculate delta g for the reaction at 298 k
Web Calculate Delta G degree for each reaction at 298k using Delta G degree f values. Web Calculate Delta G at standard state for the following reaction.

Solved Calculate Delta G 0 For This Reaction S 8 S Chegg Com
Web The value of ΔG o for the reaction N 2 g 3 H 2 g 2 NH 3 g is -3290 kJ at 298 K.

. Web Chemical Thermodynamics Gibbs Free Energy Problem Calculate ΔG at 298 K for these reactions and predict the effect on ΔG of lowering the temperature. 2Als 3C u2 aq 2Al3 aq 3C us 2 A l s 3 C u 2 a q 2 A l 3 a q. Calculate the value of ΔG in kJ at 298 K if the partial pressures of N 2 H 2.
Mg s Mg2 001 M Ag 00001 M Ag s Given. Mathrm NH_ 3 gmathrm HBr g. Web Calculate Delta G circ ΔG at 298 K for these reactions and predict the effect on Delta G circ ΔG of lowering the temperature.
Web For the reaction 2A g B g 2D gΔUo - 105kJ and ΔSo - 4410JK-1. W - Δn g RT. Web Temperature T 298 K.
Calculate rG and log K c for the following reaction at 298 K. Web Calculate ΔG Δ G and log K c K c for the following reaction at 298 K. Web At 298 K 25 C Δ G 0 Δ G 0 so boiling is nonspontaneous not spontaneous.
Web Expert Answer The reaction can be assumed as 2NO2 g -------- N2O4 g. PV work done and internal energy change ΔU Formulae. Web If we know the standard state free energy change Go for a chemical process at some temperature T we can calculate the equilibrium constant for the process at that.
Check Your Learning Use standard enthalpy and entropy data from Appendix G to. Web For which. Kp p2 SO3 p2 SO2 pO2.
Δn g moles of. CaCO3 s CaO. Web If you want to calculate DeltaG under non-standard conditions you need to use the equation DeltaG DeltaG0 RTlnQ where Q is the ratio of concentrations or.
Partial pressure of N2O4 PN2O4 160 atm. Web asked Nov 2 2018 in Chemistry by Richa 610k points edited Nov 2 2018 by Richa. Web Calculate emf and Δ G for the following cell at 298 K.
EoMg2 Mg - 237 V EoAg Ag 080 V Class. 2Al s 3Cu 2. EqN_ 2 g 3 H_ 2 g leftrightarrow 2 NH_ 3 g eq Where eqdHo -922 kJmol eq.
Web An oxidation reduction reaction has 3 moles of electrons transfered in the overall reaction. If we find the standard free energy change ΔG for this reaction we can find Kp from which we can get pSO2. Calculate ΔGo for the reaction and predict whether the reaction may occur.
The equallibrum constant K 38x10 22 Calculate delta G at 298 K. ΔH ΔU Δn g RT. 2 Mg s O_2 g rightarrow 2 MgO s BaO s CO_2 g rightarrow BaCO_3 s MnO_2 s.
Given Partial pressure of NO2 PNO2 040 atm.
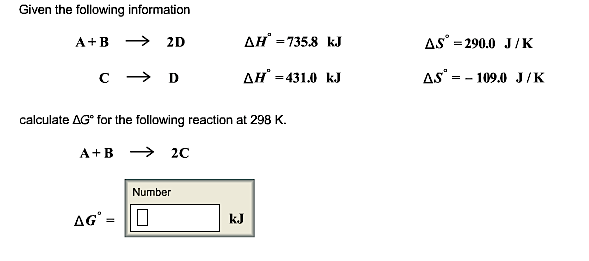
Solved Given The Following Information Calculate Delta G Chegg Com

Electronic Coupling In 1 2 3 Triazole Bridged Ferrocenes And Its Impact On Reactive Oxygen Species Generation And Deleterious Activity In Cancer Cells Inorganic Chemistry
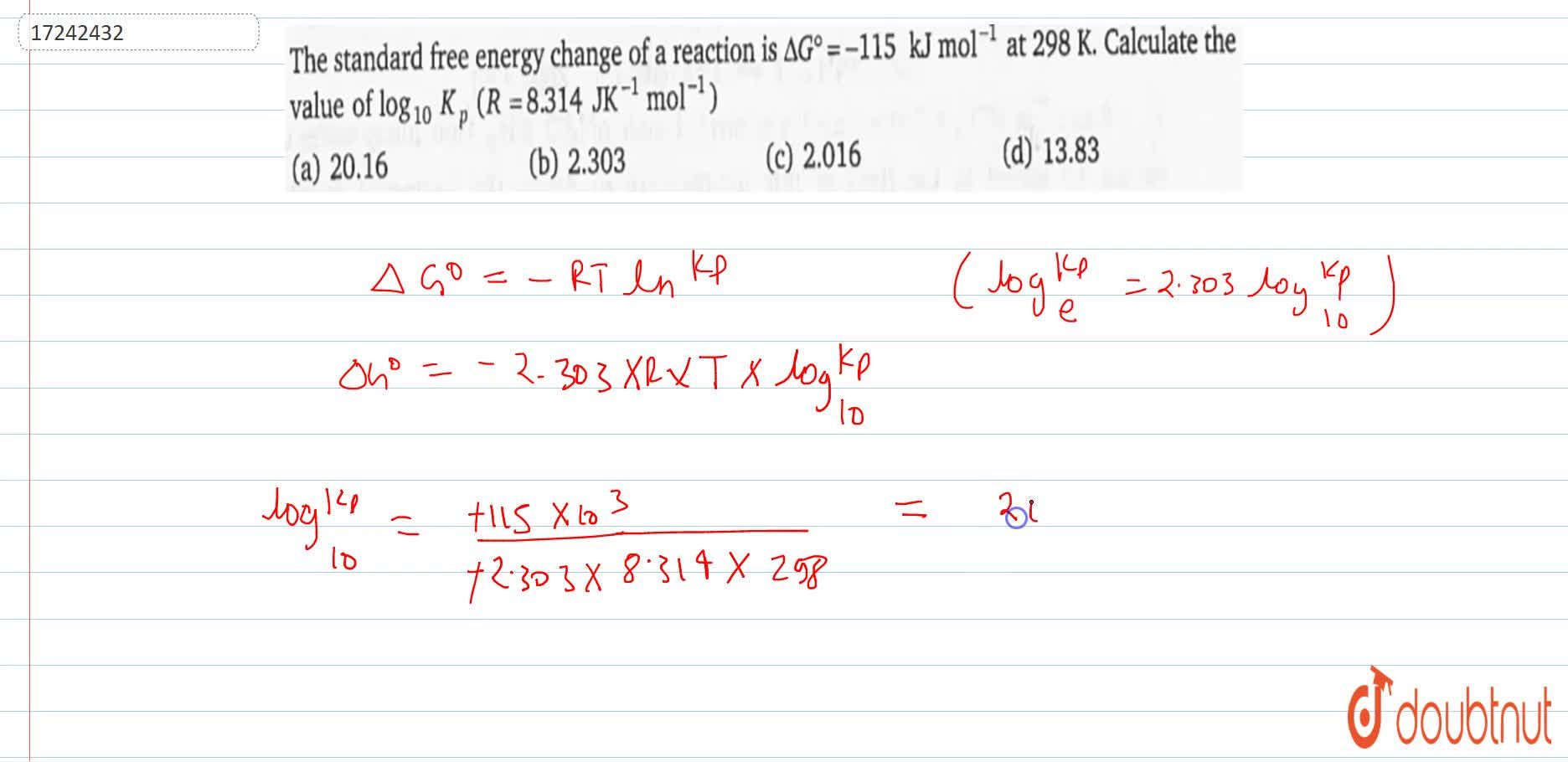
The Standard Free Energy Change Of A Reaction Is Deltag Kj Mol 1 At 298 K Calculate The Value Of Log 10 K P R 8 314jk 1 Mol 1
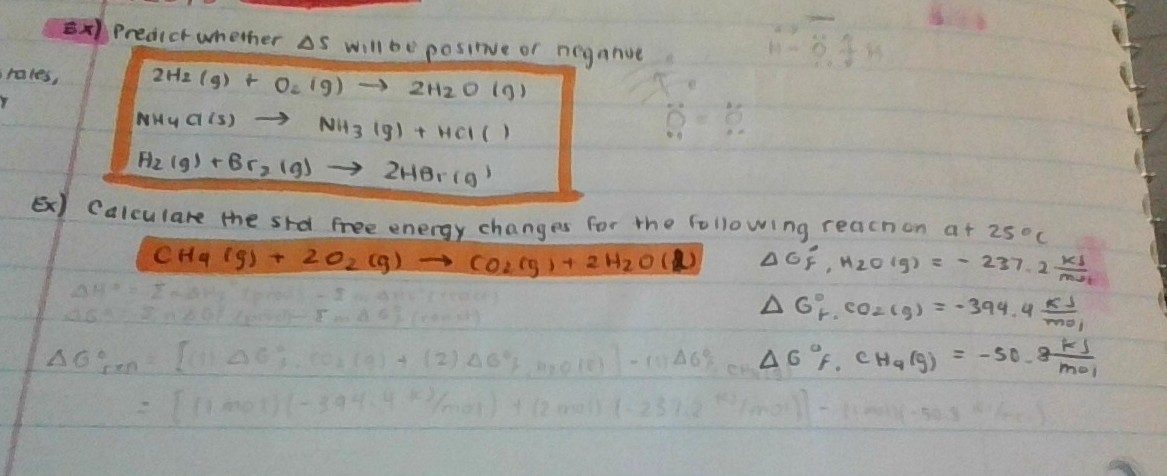
Calculate The Standard Free Energy Change For The Below Reaction At 25 Degrees Celsius Socratic

Homogeneously Catalyzed Electroreduction Of Carbon Dioxide Methods Mechanisms And Catalysts Chemical Reviews

Determine D G O For The Following Reaction Co G 12o2 G Co2 G D H O 282 84 Kj Given S O Co2 213 8 Jk 1 Mol 1 S O Co G 197 9
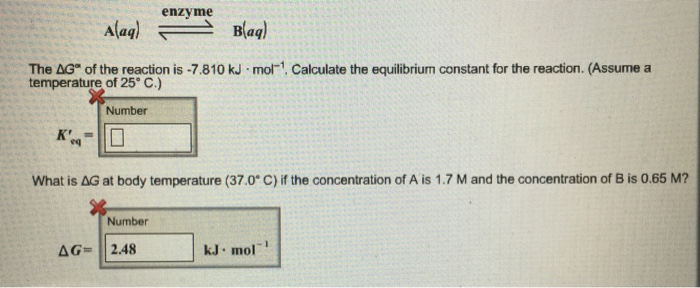
Solved The Delta G Of The Reaction Is 7 810 Kjxmol 1 Chegg Com

30 Electrochemistry Ws Pdf Electrochemistry Redox

The Value Of D G For The Reaction At 298 K Is

Pdf Modelling Heterogeneity Effects On Radionuclide Transport In The Far Field Of A Radioactive Waste Repository Nick Evans Academia Edu

Sc Hopo A Potential Construct For Use In Radioscandium Based Radiopharmaceuticals Inorganic Chemistry

Homogeneously Catalyzed Electroreduction Of Carbon Dioxide Methods Mechanisms And Catalysts Chemical Reviews

30 Electrochemistry Ws Pdf Electrochemistry Redox
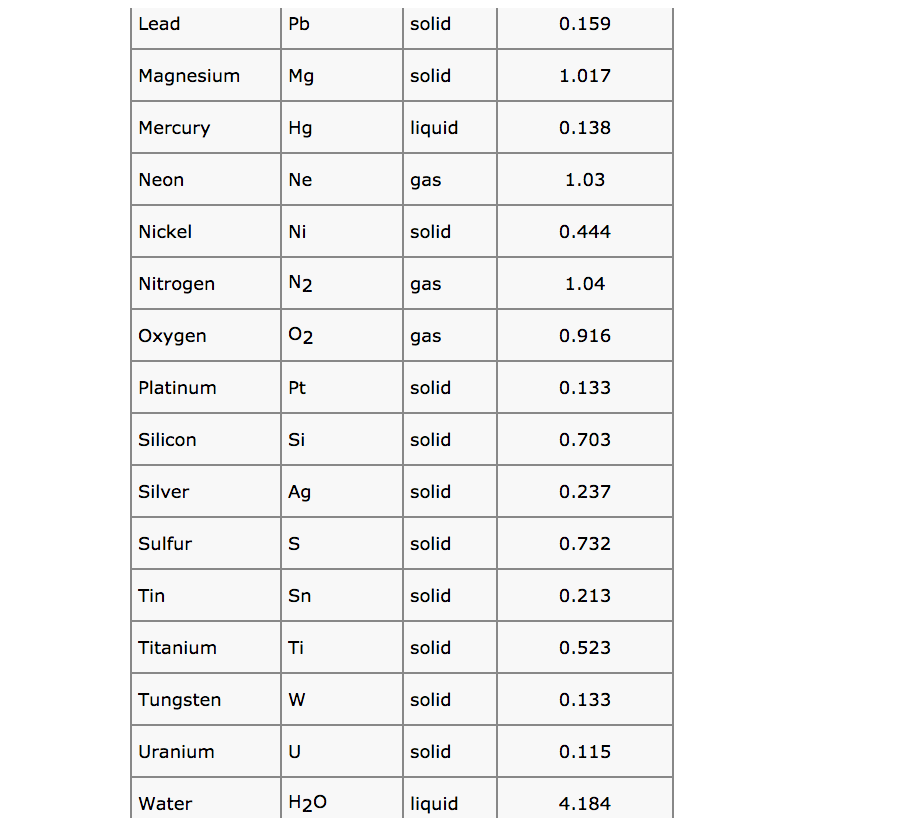
Solved Use Standard Thermodynamic Data In The Chemistry Chegg Com
Atomphysik Tagesubersichten Atomphysik Dpg Verhandlungen
Analytical Chem Istry Depauw University

Calculate The Delta G Value From The Following Data And State Whether The Process Will Be Spontaneous Or Non Spontaneous At 298k The Heat Of Reaction Is 85 0 Kj And The